Further Reading - Radio Waves
Nuclear Magnetic Resonance
Nuclear Magnetic Resonance (NMR) is the natural phenomena that MRI machines utilise. It is when a nucleus in a magnetic field absorbs and re-emits electromagnetic radiation. Nuclear magnetic spin, S, is an intrinsic property of atomic and sub-atomic particles. As protons have S = ½, they can have two discrete possible states of m = -½ or m = ½, and when there is no magnetic field they are degenerate which means that they have the same energy. As they have the same energy, there is an equal chance for the proton to be in either m = ½ or m=-½. When the proton is placed in a magnetic field, the proton’s magnetic moment interacts with the field and causes the energies of the m = -½ and m = ½ to shift so they are no longer equal (see the diagram below). As m = ½ is at a lower energy now, the proton would prefer to be in that state. The proton can absorb energy from electromagnetic waves to change into the m = -½ state, and the energy gap corresponds to the frequency of radio waves. Once the magnetic field has been turned off, the protons remain in this excited state, even though it isn’t their favoured state as it’s in a higher energy. As a result of this, they decay to a lower energy after a time, called the relaxation time, by emitting a photon with the energy that they have lost through decaying. [R2] [R4]

Graph showing how the presence of a magnetic field causes energy levels to split. Source (Wikipedia)
How to get a 3D MRI scan
As the energy shift of the states is dependent on the magnetic field strength (see above graph), you would require different frequency radio waves to excite different protons in different magnetic fields. MRI machines use two separate magnetic fields which have a gradient, meaning it is continuously varying across your body. This allows the machine to obtain location information of where the photon originated because every location in your body has a different field strength. The two separate fields are orientated so that a 3D image can be obtained. [R4]
Heat energy from EM waves
As mentioned, here, on the electromagnetic spectrum page, electromagnetic waves can have different wavelengths. The main differentiating feature, apart from the wavelength (or frequency), is that as you go from radio waves to gamma rays, the energy of the photons increase. There is a direct relation between frequency and energy which explains this: E=hf, where f is the frequency of the photon and h is a constant called Planck’s constant. When a photon is absorbed by a cell, or anything, it gets converted into energy equal to the amount given by this equation. This energy can cause ions in the cell to vibrate or could trigger a reaction in the cell. The result of the ions vibrating is that they will emit heat energy which could cause parts of the cell to breakdown by breaking bonds. [R11]
Electrons have charge, therefore they produce an electric field, and when moving, they also produce a magnetic field. This is why wires carrying current can cause compasses to deflect when placed nearby; it’s also how electro-magnets work. Electrons in a radio coil are subject to an alternating current, which means they are changing direction rapidly. This in turn means they are rapidly accelerating and decelerating. As the electron is accelerating (not just moving at a constant speed), the magnetic field produced by it will also be changing. These oscillating fields propagate at the speed of light, as shown by the image opposite, and the frequency at which they oscillate governs the frequency of the electromagnetic wave produced. [R9][R10]
Producing Radio waves
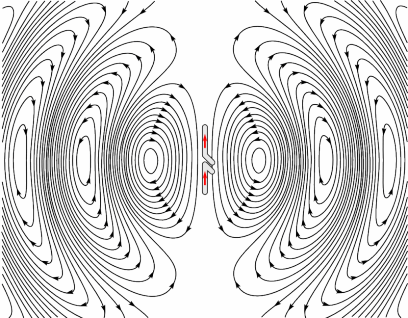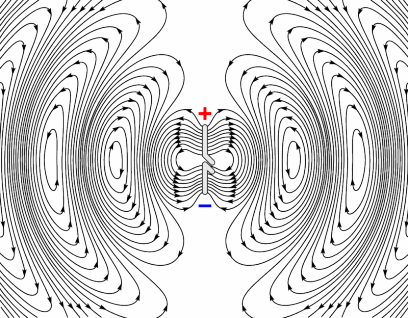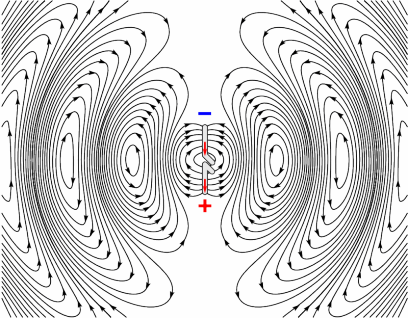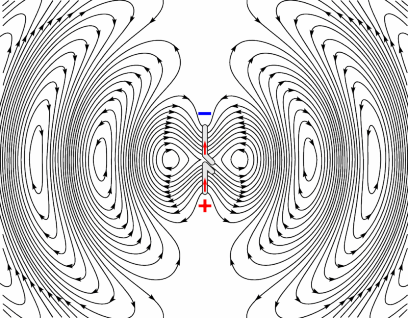
Animation showing how an antenna generates an oscillating EM field. Source (Pinterest)
